Kenvue-Philippines-Employee-Store
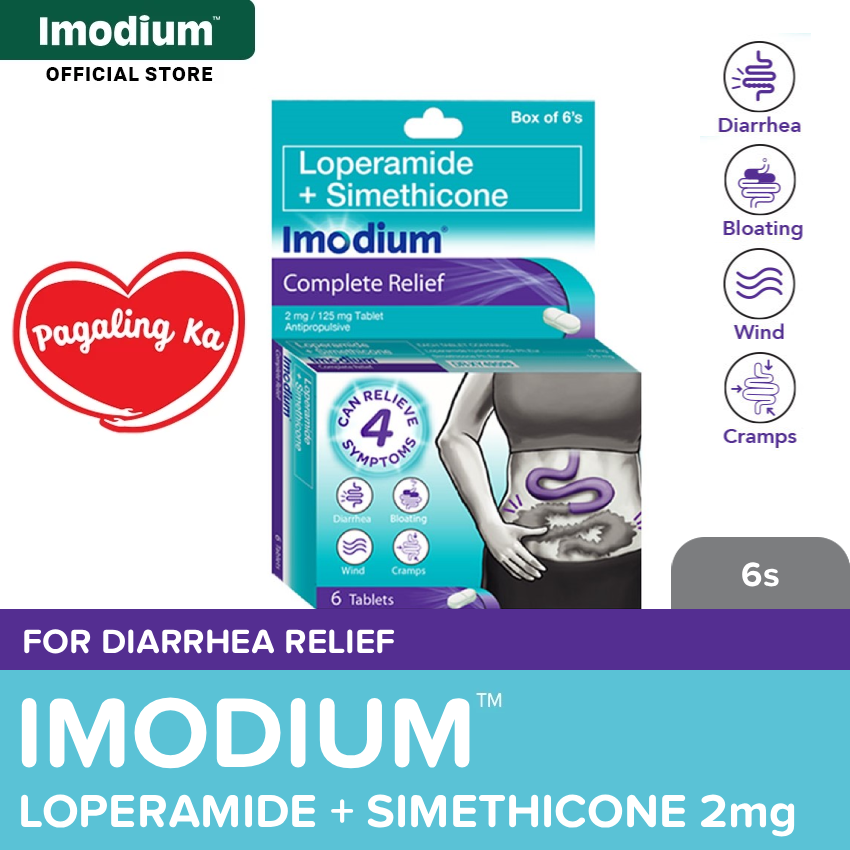
Imodium Complete Relief 2mg 6s
Imodium Complete Relief 2mg 6s
Couldn't load pickup availability
- Loperamide + Simethicone (Imodium™ Complete Relief) gives you 2x faster relief from 4 symptoms works in as fast as 1 hour vs. S. boulardii capsule
- Can relieve 4 Symptoms: Diarrhea, Boating, Cramps, and Wind.
PRODUCT DESCRIPTION
Loperamide + Simethicone (Imodium™ Complete Relief) is indicated for the control of acute diarrhea of any cause and its commonly associated symptoms. These are often attributed to “trapped wind” and include abdominal discomfort, bloating, cramping and flatulence.
DIRECTIONS FOR USE
DOSAGE & MODE OF ADMINISTRATION:
Adults and pediatrics (12 years and older)
Two tablets (4 mg) initially, followed by one tablet (2 mg) after every loose stool. Not more than 4 tablets (8 mg) should be taken in a day, limited to no more than 2 days.
Pediatrics (under 12 years of age)
Loperamide – Simethicone should not be used in children less than 12 years of age.
ELDERLY & RENAL IMPAIRMENT
No dosage adjustment is necessary in renal impairment.
Hepatic Impairment
Although no pharmacokinetic data are available in patients with hepatic impairment, Loperamide – Simethicone should be used with caution in such patients because of reduced first pass metabolism.
ABOUT THE BRAND
Nothing works faster than Imidium. Imodium contains an active ingredient called Loperamide, which works in harmony with your body to help restore the natural rhythm of your intestines, gently returning digestion to a normal pace. This allows the body to start absorbing fluids and salts as it should and to restore the consistency of stools. Nothing works faster than Imodium!
FORMULATION
Each tablet contains
Loperamide HCl, Ph Eur. 2 mg
Simethicone, Ph. Eur ……125 mg
WARNINGS & PRECAUTIONS
When should you not take this Medicine?
Loperamide-simethicone should not be used in children less than 12 years of age.
Loperamide-simethicone is contraindicated in patients with a known hypersensitivity to loperamide, simethicone or to any of the excipients.
Loperamide-simethicone should not be used as the primary therapy:
• in patients with acute dysentery, which is characterized by blood in stools and high fever,
• in patients with acute ulcerative colitis,
• in patients with bacterial enterocolitis caused by invasive organisms including Salmonella, Shigella, and Campylobacter,
• in patients with pseudomembranous colitis associated with the use of broad-spectrum antibiotics
Loperamide-simethicone should not be used when inhibition of peristalsis is to be avoided due to the possible risk of significant sequelae including ileus, megacolon and toxic megacolon. Loperamide- simethicone must be discontinued promptly when constipation, abdominal distension or ileus develop.
Care that should be taken when taking this Medicine?
Treatment of diarrhea with loperamide-simethicone is only symptomatic. Whenever an underlying etiology can be determined, specific treatment should be given when appropriate.
In patients with (severe) diarrhea, fluid and electrolyte depletion may occur. In such cases the administration of appropriate fluid and electrolyte replacement should be considered.
If clinical improvement is not observed within 48 hours, the administration of loperamide-simethicone should be discontinued. Patients should be advised to consult their physician.
Patients with AIDS treated with loperamide-simethicone for diarrhea should have therapy stopped at the earliest signs of abdominal distension. There have been isolated reports of obstipation with an increased risk for toxic megacolon in AIDS patients with infectious colitis from both viral and bacterial pathogens treated with loperamide HCl.
This medicine must be used with caution in patients with hepatic impairment as it may result in a relative overdose leading to CNS toxicity.
Abuse and misuse, as an opioid substitute, have been described in individuals with opioid addiction.
Undesirable Effects of this Medicine
Throughout this section, adverse reactions are presented. Adverse reactions are adverse events that were considered to be reasonably associated with the use of loperamide-simethicone or loperamide HCl based on the comprehensive assessment of the available adverse event information. A causal relationship with loperamide-simethicone cannot be reliably established in individual cases. Further, because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Share
-
Free Shipping
Unexpected shapes with smart details, functionality, a new luxury feel with a contemporary price point.
-
Hassle-Free Exchanges
Exchanges are free. Try from the comfort of your home. We will collect from your home, work or an alternative address.
